Discussion:
Questions on Ionic/Covalent Bonding
1. Which bonds below are polar and which are nonpolar? If a bond is polar, indicate which end is positive and which end is negative. (Pol Cov Bonds -Electronegativity )
C-S
F-C
N-O
Si-Br
Cl-C
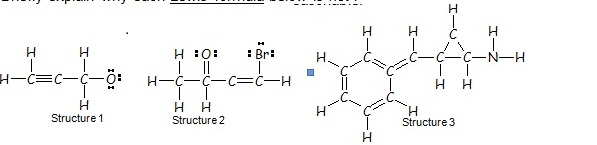
2. Briefly explain why each Lewis formula attached is not reasonable.
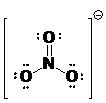
3. A typical N-O single bond is 136 pm long and a typical N=O double bond is 115 pm long. The nitrate ion (shown below) has been found to contain three nitrogen-oxygen bonds with identical bond lengths of approximately 125 pm. Explain this observation in terms of resonance structures and bond order. (Bond Length & Order)
4. The 3 resonance structures for the SCO molecule are shown in the attachment.
a) Calculate the formal charge on each atom in each structure.
b) Which Lewis formula is the most reasonable?
c) Which Lewis formula is the least reasonable?
d) Explain your answers to parts b and c in terms of electronegativity and formal charges. (Formal Charge/Lewis Formulas)
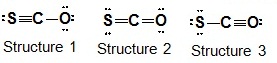
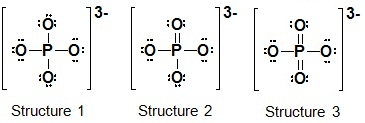
5. The phosphate ion contains a central phosphorus atom surrounded by 4 oxygen atoms. Calculate the formal charge on each atom in each structure. Which Lewis formula is the most reasonable? Which Lewis formula is the least reasonable? Explain your answers to parts and in terms of electronegativity and formal charges. (Formal Charge/Lewis Formulas)