Discussion:
Ozonolysis of Myrcene
Myrcene, C10H16, a terpene, absorbs three moles of hydrogen to form C10H22. Upon ozonolysis myrcene yields the two compounds shown in the first attached
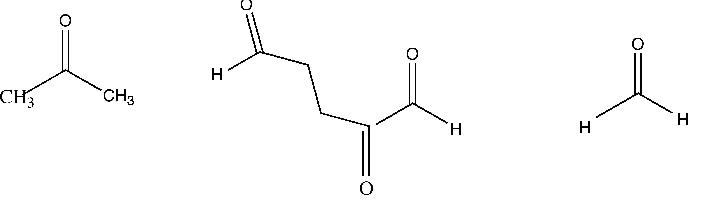
1. What structures for myrcene are consistent with the facts?
2. Based on the isoprene rule (naturally occurring terpenes are made up of isoprene segments) what is the most likely structurefor myrcene?
Dihydromyrcene C10H18, formed from myrcene absorbs 2 moles of hydrogen to form C10H22. Upon cleavage with KMnO4, dihydromyrcene yields the structures showin in.
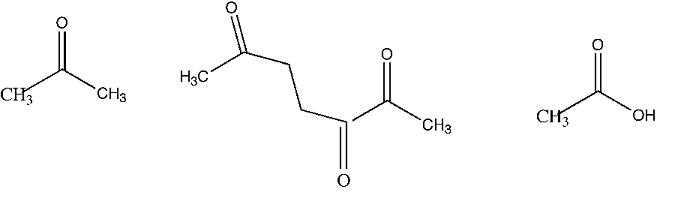
3. Keeping in mind the isoprene rule what is the most likely structure for dihydromyrcene?
4. Is it surprising that a compound of this structure is formed by reduction of myrcene? Explain