Assignment:
1. Look up the definition of the following terms in your textbook and re-write it in your own words here:
a) Heat
b) Specific heat
c) Heat capacity
2. In the three samples of identical material below, each box represents 1 gram

If 100 Joules is transferred to the Sample A, its temperature goes up by 10 degrees Celcius.
a) How many calories does each gram of Sample A receive?
b) What is the specific heat of the material?
c) If 100 Joules were transferred to Sample B what is the change in temperature? Explain how you got your answer
d) If 100 Joules were transferred to Sample C what is the change in temperature? Explain how you got your answer
3. 500 joules are transferred from Sample D to Sample E.
• Sample D has a specific heat of 10 J/g/C
• Sample E has a specific heat of 25 J/g/°C.
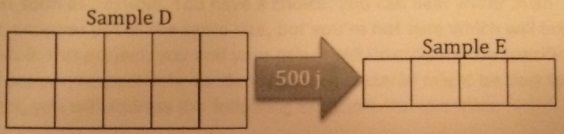
a. How much heat does each gram of Sample D lose?
b. How much does the temperature of each gram of Sample D change by?
c. How much does the temperature of the entire Sample D change by?
d. How much heat does each gram of Sample E gain?
e. How much does the temperature of each gram of Sample E change by?
f. How much does the temperature of the entire Sample E change by?
4. An aluminum can with a mass of of 80.0 g is holding 500.0g of water. The can & water are at 20 degrees Celsius. How much heat must be transferred to the can & water to increase its temperature to 80 degrees Celsius?