Assignment:
Problem 1: A soap bubble in the sunlight shows a rainbow of colors at different points on the bubbles surface. What is the change in thickness of the soap bubble from one band of green to the next band of green? Assume green light has a wavelength of 500 nm, and the index of refraction for the soap film is nsoap =1.43

Problem 2: You are given a bucket containing 2.6 kg of water at room temperature (20° C) and a 5.4 kg block of very cold aluminum. What should the initial temperature of the aluminum block be in order to (a) reduce the temperature of the water to 0° C and (b) freeze the water to solid ice at 0°C?
Problem 3: Air (which we can treat as an ideal gas) that initially occupies 1.4 m3 at a pressure of 9.80 x 104 Pa is compressed isothermally to a pressure of 1.28 x 105 Pa.
(a) How much work is done by the air during the isothermal compression? The air is then cooled at constant pressure until it occupies the initial volume of 1.4 m3 .
(b) How much work is done by the air during the constant pressure cooling?
( c ) How much work is done by the air during the total process (isothermal compression followed by constant pressure cooling)?
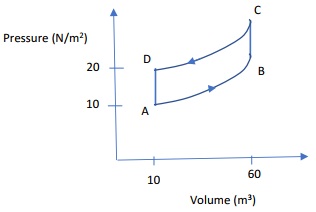
Problem 4: An ideal gas is taken through the complete cycle A-B-C-D-A as shown below in the p-V plot. Calculate the work done by the gas. Note, you may assume that the path A-B has the same slope and curvature as the path from D-C
Problem 5: A container of hydrogen gas and a container of nitrogen gas are brought into thermal equilibrium with each other at some temperature T. The average kinetic energy of a hydrogen molecule is KH2 = 2.34 x 10-17 J. What are (a) the temperature of the nitrogen (b) the rms average velocity of the nitrogen molecules. You may assume that the nitrogen molecules are 14 times more massive than the
hydrogen molecules.
Problem 6: Consider the cooling of 100 g of water from 20°C to 0°C. (a) What is the change in the entropy Delta S of the water? If the cooling is done with a reverse Carnot engine, with coefficient of performance 4.30, how much (b) heat is delivered to the room?