Discussion:
Electrons Table
1- What is a likely source of error in identifying a compound using thin layer chromatography? Choose from: not accurately measuring the volume, the time, mass, distance or density
2- What property is used to separate solutes using thin layer chromatography?
3- Consider the following image, in which the 'x' designates where the solute was spotted. What is the Rf value of the solute? Choose from: 0.36, 0.75, 0.70,0.67, 1.20
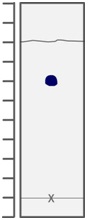
4- Which of the following atoms would be expected to have the largest radius? Choose from : B, Ne,C,,O Li
5- Which of the following atoms will have no unpaired electrons? Choose from : Br, Be, Si, Na, Al
6- What is the complete ground state electron configuration for oxygen (O)?
7- Which of the following elements would you expect to have the lowest first ionization energy? Choose from: He,Ar,Na,Xe,Ne
8- Write a Lewis structure for each molecule or ion. Draw the molecule by placing atoms on the grid and connecting them with bonds.
Include all lone pairs of electrons.
N2H2
N2H4
CN-
NO -