Assignment:
Problem 1. The 22Na atom has a mass-energy of 20487.686 MeV and it decays, with a half-life of 2.6 years, from its 3+ ground state by β+ emission and electron capture (EC) to the 2+ first excited state of 22Ne (atomic mass-energy = 20484.844 MeV). (a) Write down expressions for the two types of decay; (b) calculate the β+ decay Q value in MeV; (c) calculate the EC decay Q value in MeV.
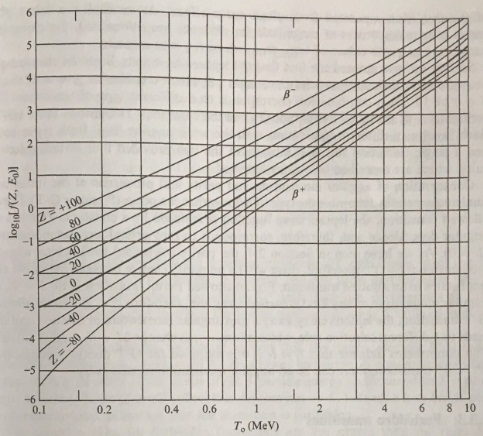
Problem 2. Show that the Q value for the β decay of the neutron is 0.782 MeV. Give the type of transition and, hence, obtain an estimate of the half-life. (Hint: you will require the approximate value of log10 f for this transition, which can be found from Figure.)