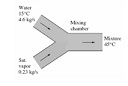
Solve the below problem:
Q: Liquid water at 15°C is heated in a chamber by mixing it with saturated steam. Liquid water enters the chamber at the steam pressure at a rate of 4.6 kg/s and the saturated steam enters at a rate of 0.23 kg/s. The mixture leaves the mixing chamber as a liquid at 45°C. If the surroundings are at 15°C, determine
(a) the temperature of saturated steam entering the chamber,
(b) the energy destruction during this mixing process, and
(c) the second-law efficiency of the mixing chamber.