Assignment:
The beryllium (3) ion, Be 3+ is like the hydrogen atom in that it has a single electron. Its emission line spectrum is similar to that of hydrogen, except that the value of the Rydberg constant is different. The following lines were observed for a 'series' (i.e. all have the same value of nf) the
Be 3+: 117.18 nm, 80.10nm, 68.35nm, 62.80nm.
a. Show that these follow the relationship
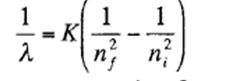
b. Find the values of nf for the series and ni for each line
c. Find a relationship between K and Rf, the Rydberg constant for hydrogen. To what property is beryllium is this connected?