Assignment:
Question 1. The isomerization of cyclopropane to form propene
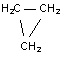
→CH3 - CH =CH2CH2
is a first-order reaction. At 760 K, 15 percent of a sample of cyclopropane changes to propene in 6.8 minutes. What is the half-life of cyclopropane at 760 K?
Question 2. Consider the two gaseous equilibria (K1 and K2):
SO2(g) + ½O2(g) ↔SO3(g) K1
2SO3(g) ↔2SO2(g) + O2(g) K2
The values of the equilibrium constants K1 and K2 are related by ________.
Question 3. The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (i.e., least completion →greatest completion).
- 2NOCl ↔2NO + Cl2 KP = 1.7 x 10-2
- N2O4 ↔2NO2 KP = 1.5 x 103
- 2SO3 ↔2SO2 + O2 KP = 1.3 x 10-5
- 2NO2 ↔2NO + O2 KP = 5.9 x 10-5
Question 4. On analysis, an equilibrium mixture for the reaction
2H2S(g) ↔2H2(g) + S2(g)
was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant for this reaction.
Question 5. At 35oC, the equilibrium constant for the following reaction is Kc = 1.6 x 10-5.
2NOCl(g) ↔2NO(g) + Cl2(g)
An equilibrium mixture was found to have the following concentrations of Cl2 and NOCl:
[Cl2] = 1.2 x 10-2 M; [NOCl] = 2.8 x 10-1 M. Calculate the concentration of NO(g) at equilibrium.
Question 6. For the following reactions the equilibrium constants are defined.
A + 2B ↔C K1
C ↔D + E K2
Then for the reaction
A + 2B ↔D + E Kc
the equilibrium constant must be equal to ________.
Question 7. At 700 K, the reaction
2SO2(g) + O2(g) ↔2SO3(g)
has an equilibrium constant Kc = 4.3 x 106, and the following concentrations are present:
[SO2] = 0.10 M
[SO3] = 10 M
[O2] = 0.10 M
Is the mixture at equilibrium? If not at equilibrium, in which direction-left to right or right to left- will the reaction occur to reach equilibrium?
Question 8. For the following reaction at equilibrium, which choice gives a change that will shift the position of equilibrium to favor formation of more products?
2NOBr(g) ↔2NO(g) + Br2(g) ΔHorxn = 30 kJ
Provide complete and step by step solution for the question and show calculations and use formulas.