Assignment:
Question 1. Give the Frequency ranges (in cm-1) for each of the following:
a. O-H stretch
b. aldehyde
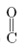
c. –C-H stretch
d. =C-H stretch
Question 2. Briefly describe how could you distinguish between cyclohexane and cyclohexene by IR?
Question 3. Define the following terms:
a. Coupling constant
b. Chemical shift
c. Spin-spin coupling.
Question 4. A solid samples are prepared by examination by IR spectroscopy by fusing them in a KBr pellet or dissolving them in CCl4 and subtracting a background of pure CCl4 from the spectrum. Assuming equal solubility of the compound in both solvents why would acetone be a poor substitute for CCl4 in the latter method.
Question 5. Predict the splitting pattern for each kind of proton in the 1H NMR spectra of the following:
a. CH3CH2CH2Br
b.
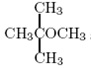
Question 6. Predict the number of signals that would appear in the 13C NMR spectra of the molecules from the previous questions.
Question 7. Propose a structure for a molecule with a molecular formula C10H13NO and the following 1H NMR data. Explain your answer.

Provide complete and step by step solution for the question and show calculations and use formulas.