Assignment:
Q1. Why a polymer such as polybutadiene can be cross-linked, however a polymer such as polyethylene cannot be cross-linked. Please make reference to what it is about the chemistry of the repeating units that allows for the formation of cross-links.
Q2. Car tires are made of vulcanized rubber, which is a crosslinked polymer formed through the addition of sulfur and heat to form disulfide bridges between polymer chains. Car tires are actually fairly difficult to recycle when compared to other non-crosslinked polymer products such as plastic bottles. Consider why this might be the case, and give your thoughts.
Q3. Shown is a schematic representation of the water-ethanol phase diagram.
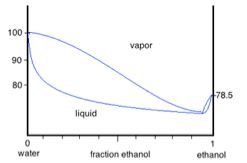
Making reference to the phase diagram, explain in a few sentences how one could enrich a solution of 15 percent ethanol - 85 percent water to a composition of 60 percent ethanol - 40 percent water. Be qualitative in your description, not quantitative.
Furthermore, explain why this procedure could not be used to reach a completely pure composition of 100 percent ethanol.