Assignment:
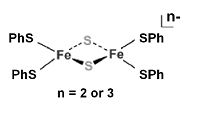
Q1. Comment on the applicability of [Fe2(S)2(SPh)4]^n shown below as a model for redox/spin states of [2Fe*2S] ferredoxin, given that the observed values of the effective magnetic moments are 0 and 1.75 for [Fe2(S)2(SPh)4]^-2 and [Fe2(S)2(SPh)4]^-3respectively
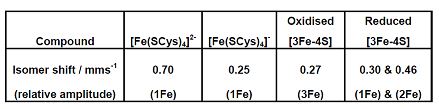
Q2. Explaining your reasons, use the ^57Fe Mossbauer data below to answer the following questions
i) Calculate the average oxidation state of iron in the two [3Fe-4S] ferredoxins
ii) In both cases calculate the overall charge on the [3Fe-4S] core
iii) Also with the aid of diagram rationalize the s = 2 spin state of the reduced 3Fe-4S] ferredoxin
Q3. For a [4Fe - 4S] ferredoxin, the following series of redox reactions, shown, are possible
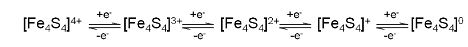
i) Which of these redox couples are accessible under physiological conditions?
ii) Which redox couple represents the high-potential iron-sulfur protein system?
iii) Also, what structure changes would occur to the (Fe4S4) core during these electron transfer processes?
Provide complete and step by step solution for the question and show calculations and use formulas.