Assignment:
Q1. Give the structures of all possible products when 3-bromopentane reacts by the E2 mechanism.
Q2. Ethers and alcohols can be isomeric. Write the structures, and give names for all possible isomers with the molecular formula C4H10O.
Q3. Using a Grignard reagent and the appropriate aldehyde or ketone, show how each of the following can be prepared.
a. 2-pentanol b. 3-phenyl-3-hexanol
Q4. Give the structure of each product
a.
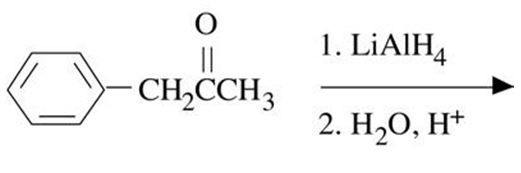
b. CH3CH2CH2CH2OH
Jones’
→
Reagent
c. 2-butanone + HCN
Q5. In each of the following pairs of acids, which would be expected to be stronger, and why?
a. p-BrC6H4CO2H or m-BrC6H4CO2H
b. b. CH3CH2CHBrCO2H orCH2BrCH2CH2CO2H
Q6. Write out all the steps in the mechanism for ammonolysis of butyl benzoate.
Q7. Complete the following equations.
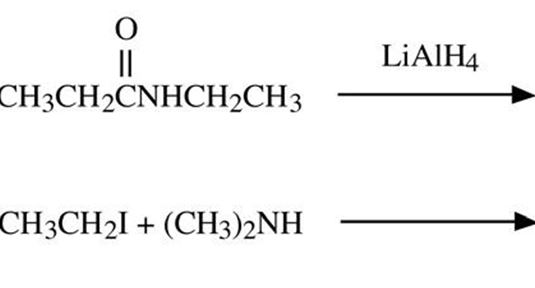
Q8. Tell which is the stronger base and why: ammonia or dimethylamine?
Provide complete and step by step solution for the question and show calculations and use formulas.