Assignment:
Question 1. Briefly explain what is wrong with the following orbital diagrams.
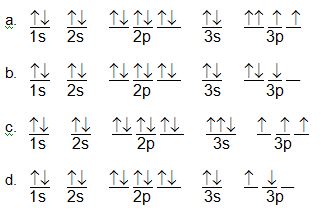
Question 2. Briefly explain what is wrong with the following electron configurations.
a. 1s22s22p53s23p3
b. 1s22s22p63s13p4
c. 1s22s22p63s23p7
d. 1s22s22p63s23p63d2
Question 3. (Periodic Trends: Atomic Radius)
a. As you go down a column in the periodic table, atomic size increases. Explain why in terms of principle quantum number and effective nuclear charge.
b. As you go left-to-right across a row in the periodic table, atomic size decreases. Explain why in terms of principle quantum number and effective nuclear charge.
Question 4. (Periodic Trends: Ionization Energy)
a. As you go down a column in the periodic table, ionization energy decreases. Explain why in terms of principle quantum number and atomic radius.
b. As you go left-to-right across a row in the periodic table, ionization energy increases. Explain why in terms of effective nuclear charge and atomic radius.
Question 5. (Periodic Trends: Electron Affinity)
a. As you go down a column in the periodic table, electron affinity becomes less negative. Explain why in terms of principle quantum number and atomic radius.
b. As you go left-to-right across a row in the periodic table, electron affinity becomes more negative. Explain why in terms of effective nuclear charge and atomic radius.