Assignment:
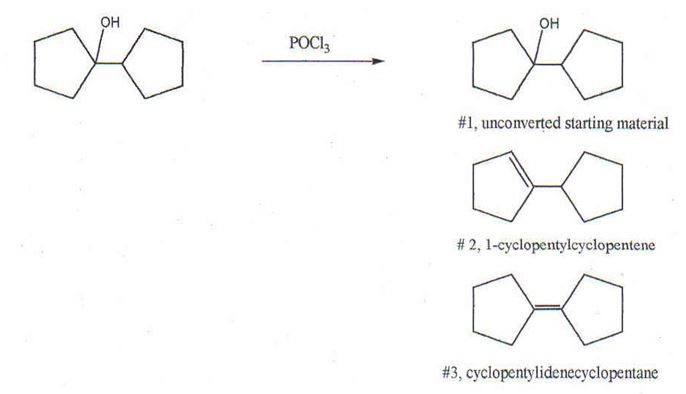
Q1. In this problem the tertiary alcohol below was subjected to dehydration with phosphorous oxychloride. The potential products are shown below. The product was isolated and purified by distillation. An NMR of the major product exhibited two triplets only, one centered at 2.1δ and the other at 0.9δ . Indicate the product that seems to have been produced. Explain the spectrum.
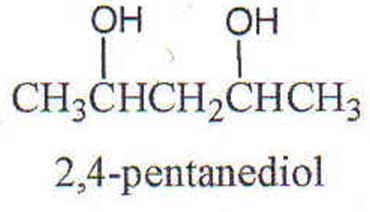
Q2. In this problem a C NMR spectrum of 2,4-pentanediol shows five peaks at 23.3δ, 23.9δ, 46.5δ, 64.8δ, and 68.1δ. Symmetry in the structure is obvious. Why does the NMR show five peaks instead of three?
Provide complete and step by step solution for the question and show calculations and use formulas.