Assignment:
Q1. Use the Lewis structure form to show the structures of the element F2 and N2 and the compounds NaCl, NH3 and H2O. Write out the molecules with stick formulae.
Q2. Use Lewis structures for the elements and so suggest a structure for the compound CCl2F2 called Freon-12.
Q3. Suggest why sodium exists as Na+ and not Na2+ in its compounds.
Q4. In water molecules the O atom attracts the electrons in the H-O bonds more strongly than the H atom does. What effect will this have on the position of the electron pair in each of the H-O bonds? Then explain how this accounts for water being a liquid at normal temperatures, rather than a gas (as for such a small molecule).
Q5. A molecule of glucose has the structure. Try to explain why this compound is so soluble in water.
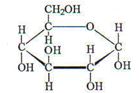
Provide complete and step by step solution for the question and show calculations and use formulas.