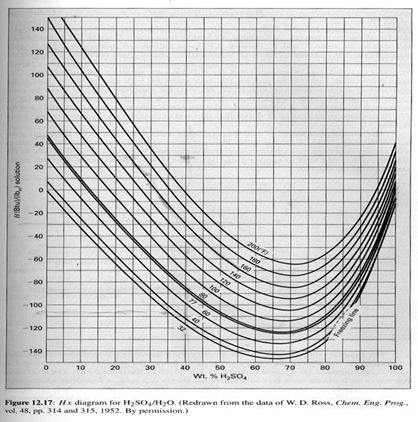
Discussion:
From Introduction to Chemical Engineering Thermodynamics, Smith, Van Ness, & Abbott, Sixth Edition
Q: An insulated tank, open to the atmosphere, contains 1,500 (lbm) of 40-wt-% sulfuric acid at 60oF. It is heated to 180oF by injection of live saturated steam at 1 atm, which fully condenses in the process. How much steam is required, and what is the final concentration of H2SO4 in the tank?