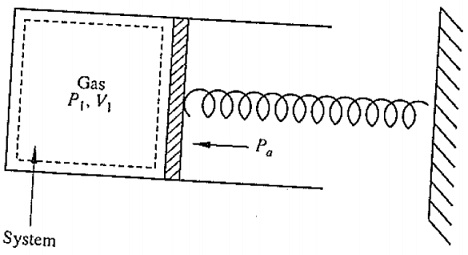
Question 1: Consider the system shown below. Initially, the gas is at 200 kPa and occupies a volume of 0.1 m3. The spring exerts a force which is proportional to the displacement from its equilibrium position. The atmospheric pressure of 100k Pa acts on the other side of the piston. The gas is heated until the volume is doubled and the final pressure is 500 kPa. Calculate the work done by the gas.
Question 2: One kmol of ethylene is contained in a 0.6 m3 steel vessel immersed in a constant temperature bath at 200°C. Calculate the pressure developed by the gas by each of the following: (a) Ideal gas law, (b) van der Waals equation and (c) Redlich-Kwong equation.
Question 3: At the start of the compression stroke in an Otto engine the fuel air mixture is at a pressure of 100 kPa and at a temperature of 300 K. The fuel air mixture is compressed to a pressure of 2 MPa by an adiabatic and reversible process. Assuming the fuel air mixture to behave as a ideal gas with γ = 1.4, calculate the final temperature, the compression ratio which is defined as the ratio of the initial volume to the final volume, and the work done per mole of the fuel air mixture.
Question 4: Ten moles of an ideal gas with γ = 1.4, is compressed reversibly and adiabatically from 100 kPa and 27C to 1 MPa. Determine the work done on the gas, the change in the internal energy and the final temperature of the gas.
Question 5: Energy is transferred as heat by conduction from a reservoir at 480 K to a reservoir at 300 K at the rate of 50 kJ/min. Evaluate Φ(dQ/dT). What will be Φ(dQ/dT) if a reversible heat engine is operated between these two reservoirs? How much work would be done by the engine?