Discuss the below:
Q1. Dry ice is frozen carbon dioxide. If you have 1.8 kg of dry ice, what volume will it occupy if you heat it enough to turn it into a gas at a temperature of 20°C?
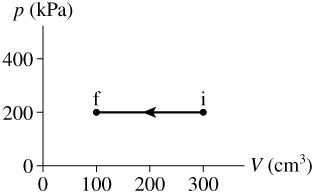
Q2. How much work is done on the gas in the process shown in the figure?
Q3. If a person has a dangerously high fever, submerging her in ice water is a bad idea, but an ice pack can help to quickly bring her body temperature down. How many grams of ice at 0°C will be melted in bringing down a 68 kg patient's fever from 40°C to 39°C?
Q4. Seals may cool themselves by using thermal windows, patches on their bodies with much higher than average surface temperature. Suppose a seal has a 0.030 m^2thermal window at a temperature of 30°C. If the seal's surroundings are a frosty -20°C, what is the net rate of energy loss by radiation? Assume an emissivity equal to that of a human.
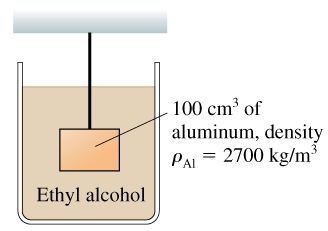
Q5. What is the tension in the string in the figure?