Question 1: What is the dominant species (H2A, HA-, A2-) of a diprotic acid with pKa1 = 3.5 and pKa2 = 6.5 at a pH of 4.8?
Question 2: Draw a Jablonski diagram. Describe the difference between the processes leading to fluorescence and phosphorescence.
Question 3: Why is Raman spectroscopy using a visible light-range laser, whereas the energy of the excited vibrations lies in the infrared energy region?
Question 4: X-ray absorption spectroscopy measures the absorption of X-rays by core electrons. What happens to excited core electrons? Where will you find the absorption edge of Mn 1s electrons, compared to that of Ni 1s electrons?
Question 5: Describe the general composition of a chromatography instrument. Give examples for each component for an instrument that allows analyte separation by vapor pressure.
Question 6: What is the separation principle in size-exclusion chromatography? In which order are analyte being eluted?
Question 7: What is the purpose of isoelectric focusing in electrophoresis?
Question 8: You measure a cell potential of -45mV for a WE of Ag/Ag+ in a silver ion solution of unknown concentration against a RE of Ag/Ag+ with a silver ion concentration of 0.5M. Determine the unknown Ag+ concentration.
Question 9: Identify the Miller indices for the plane drawn into the cubic structure below:
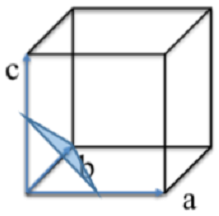
Question 10: Given a unit cell parameter a = 4.0Å and an X-ray energy of 3 keV, calculate the diffraction angle θ for the (1 0 0) plane.
Question 11: What kind of light source is used in dynamic light scattering? Which phenomenon leads to a time-dependence in the measurement?
Question 12: Describe the difference between back scattered electrons and secondary electrons. Focus on the mechanism of signal formation and the difference in information that these two signals provide.
Question 13: You need to determine the relative amount of proline in a mixture of amino acids. Which technique do you choose and why?
Wait no more and approach our Separation Principle in Size-Exclusion Chromatography Assignment Help service to secure higher grades, without putting so much effort.
Tags: Separation Principle in Size-Exclusion Chromatography Assignment Help, Separation Principle in Size-Exclusion Chromatography Homework Help, Separation Principle in Size-Exclusion Chromatography Coursework, Separation Principle in Size-Exclusion Chromatography Solved Assignments, Jablonski Diagram Assignment Help, Jablonski Diagram Homework Help, X-ray Absorption Spectroscopy Assignment Help, X-ray Absorption Spectroscopy Homework Help, Miller indices Assignment Help, Miller indices Homework Help