Film Mass Transport:
Question: Sulfur trioxide (SO3) is manufactured by the gas-phase oxidation of SO2 over a platinum catalyst:
SO2 + 1/2 O2 → SO3
The catalyst is a non-porous extrudate with the platinum deposited on the outside surface. The following data have been measured for the particle rate of reaction as a function of SO2 concentration in the bulk gas at 450 °C
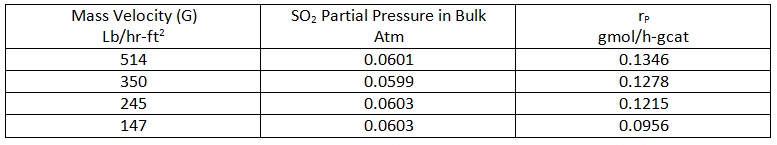
The following data apply to this problem:
? B (void fraction) = 0.43
Catalyst = 1/8 x 1/8 inch (diameter x length) extrudates (Pt on surface only)
At (specific external surface area of catalyst) = 5.12 ft2/lb
DSO2/air = 1.1 ft2/h
μ air = 0.09 lb/hr-ft
ρair = 0.0304 lb/ft3
Without calculating anything, what can you tell about the importance of film mass transport on this reaction? Explain briefly.
Question: Explain why mass transfer resistance reduces the global rate more at higher temperature than at lower temperature. Assume no heat transfer resistances are present.
Question: A gas-phase catalytic reaction is taking place in a Packed Bed Reactor (PBR). The system is isothermal but film mass transfer resistances are important.
a) Would increasing the turbulence in the gas phase increase or decrease the global rate?
b) If the system is not isothermal and the reaction is exothermic would increasing the turbulence increase or decrease the global rate?
Question: Experimental global rate data for the oxidation of SO2 over a non-porous platinum catalyst are given in the table below for two levels of conversion of SO2. Estimate the importance of film mass transport from these data by calculating the concentration difference (for SO2) between the bulk gas and the catalyst surface.
DATA:
a) Packed-bed reactor (PBR); catalyst consists of 1/8 x 1/8-inch (radius x length) tablets
b) Packing void fraction (εB) = 0.36
c) Superficial mass velocity (G) = 147 lb/hr-ft2
d) Pressure = 790 mm Hg; Temperature (assume isothermal) = 480 °C
e) Bulk gas concentration: 6.42 mol% SO2 and 93.58 mol% air
f) Specific external surface area of catalyst (am) = 5.12 ft2/lb
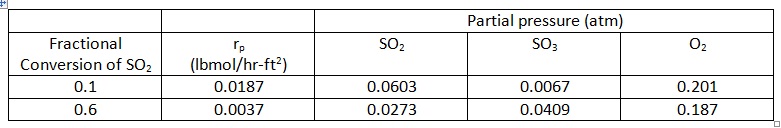
rp = particle rate of reaction (rate per unit external surface area of catalyst)
Question: Cumene (C) is catalytically cracked to manufacture benzene (B) and propylene (P). The following non-stoichiometric equation illustrates the chemistry:
C → B + P
Typical operating conditions for this reaction are a temperature and total pressure of 362 °C and 1.0 atm. respectively. A measurement of the global rate of reaction was made in the laboratory, resulting in the following value for the rate of disappearance of cumene:
rp = 76.5 kmol/m2 – h
From the data supplied, is there any evidence of either heat or mass transfer limitations for this reaction? Be as quantitative as possible in your explanation.
Assumptions:
The catalyst particle is non-porous. All thermo physical properties (density, viscosity, thermal conductivity, etc.) of the bulk gas and gas in the film can be assumed to be constant.
Data:
Average MW of gas = 34.37 kg/kmol
Gas density = 0.66 kg/m3
Gas viscosity = 0.094 kg/m – h
Gas thermal conductivity = 0.037 kcal/m – h - °C
Gas heat capacity = 33.0 kcal/kg – °C
G (mass velocity) = 56,470 kg/m2 – h
at = am = 45 m2/kg cat (specific external surface area of catalyst)
εB (bed void fraction) = 0.5
dp (catalyst particle diameter, equivalent sphere) = 0.1 cm
ΔHr (heat of reaction) = +41,816 kcal/kmol (endothermic)
Ea (activation energy for reaction) = 40 kcal/gmol
ρB (catalyst bulk density) = 5x105 g/m3
Sc (Schmidt number) = 1.483