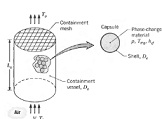
Discuss the below in detail:
Q: Latent heat capsules consist of a thin-walled spherical shell within which a solid-liquid, phase-change material (PCM) of melting point T mp and latent heat of fusion hsf is enclosed. As shown schematically, the capsules may be packed in a cylindrical vessel through which there is fluid flow. If the PCM is in its solid state and Tmp < ti,="" heat="" is="" transferred="" from="" the="" fluid="" to="" the="" capsules="" and="" latent="" energy="" is="" stored="" in="" the="" pcm="" as="" it="" melts.="" conversely,="" if="" the="" pcm="" is="" a="" liquid="" and="" tmp=""> Ti, energy is released from the PCM as it freezes and heat is transferred to the fluid. In either situation, all of the capsules within the packed bed would remain at T mp through much of the phase change process, in which case the fluid outlet temperature would remain at a fixed value To.
Consider an application for which air is chilled by passing it through a packed bed (?L = 0.5) of capsules (Dc = 50 mm) containing an organic compound with a melting point of Tmp = 4°C. The air enters a cylindrical vessel (Lv = Do = 0.40 m) at Ti = 25°C and V = 1.0 m/s.
(a) If the PCM in each capsule is in the solid state at T mp as melting occurs within the capsule, what is the outlet temperature of the air? If the density and latent heat of fusion of the PCM are p = 1200 kg/m3 and hsf = 165 kJ/kg, what is the mass rate (kg/s) at which the PCM is converted from solid to liquid in the vessel?
(b) Explore the effect of the inlet air velocity and capsule diameter on the outlet temperature.
(c) At what location in the vessel will complete melting of the PCM in a capsule first occur? Once complete melting begins to occur, how will the outlet temperature vary with time and what is its asymptotic value?