ASSIGNMENT:
Problem 1: Consider the following reaction sequence.
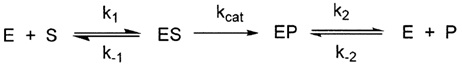
a. What is (are) the active intermediate?
b. Provide a rate law for the product.
Problem 2: A second order reaction where A → B occurs in a CSTR under isothermal conditions. The exothermic reaction occurs at 175°C. A heat exchange fluid at 25°C is available. The temperature of the reactants entering the reactor is 50°C and the heat of reaction is -15000 J/mol. The reaction rate constant is 0.005 m3/(mol•s). The heat capacity of A is 150 J/(mol•K) and its fractional conversion is 75%. The initial molar concentration of A is 300 mol/m3.
a. What is the initial molar flow rate of A if the volume is 10 m3?
b. What is the heat transfer area if the heat transfer coefficient is 1.5 KJ/(m2•°C•h)?
Problem 3: Discussion Questions. Be thorough. Write your answers.
a. Explain the design of a semi-batch reactor. What is the main advantage of using a semibatch reactor?
b. Explain the importance of porous catalyst.