Solve the below problem:
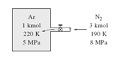
Q: A rigid tank contains 1 kmol of Ar gas at 220 K and 5 MPa. A valve is now opened and 3 kmol of N2 gas is allowed to enter the tank at 190 K and 8 MPa. The final mixture temperature is 200 K. Determine the pressure of the mixture, using
(a) The ideal-gas equation of state and
(b) The compressibility chart and Dalton's law.