Assignment:
Question 1. Balance the following redox equations by the ion-electron method:
H2O2 + Fe2+ ---> Fe3+ + H2O (in acidic solution)
CN- + MnO4- ---> CNO- + MnO2 (in basic solution)
Question 2. Calculate the standard emf of a cell that uses the Mg/Mg2+ and Cu/Cu2+ half cell reactions at 25 deg C. What is the equation for the cell reaction that occurs under standard-state conditions?
Question 3. If 2.50 g of CuSO4 are dissolved in 9.0 x 102 mL of 0.30 M NH3, what are the concentrations of Cu2+, Cu(NH3)2+4, and NH3 at equilibrium?
Question 4. In the complex ion [Fe(CN)6]4-, the oxidation number of Fe is_____??
Question 5. Consider an electrochemical cell constructed from the following half cells, linked by an external circuit and by a KCl salt bridge:
an Al(s) electrode in 1.0 M Al(NO3)3 solution
a Pb(s) electrode in 1.0 M Pb(NO3)2 solution
What is the balanced overall (net) cell reaction?
- Pb(s) + Al3+(aq) Pb2+(aq) + Al(s)
- 3Pb(s) + 2Al3+(aq) 3Pb2+(aq) + 2Al(s)
- 3Pb2+(aq) + 2Al(s) 3Pb(s) + 2Al3+(aq)
- Pb2+(aq) + Al(s) Pb(s) + Al3+(aq)
Question 6. Calculate the cell emf for the following reaction:
2Ag+(0.010 M) + H2(1 atm) 2Ag(s) + 2H+ (pH = 10.0)
Question 7. Calculate the value of Eocell for the following reaction:
2Au(s) + 3Ca2+(aq) 2Au3+(aq) + 3Ca(s)
Question 8. In the Mond process for the purification of nickel, CO is passed over the metallic nickel to give Ni(CO)4:
Ni(s) + 4CO(g) ←→ Ni(CO)4(g)
Given that the standard free energies of formation of CO(g) and Ni(CO)4(g) are -137.3 kJ/mol and -587.4 kJ/mol, respectively, calculate the equilibrium constant of the reaction at 80 Degrees C. (Assume Gfo to be independent of temperature)
Question 9. Which is the systematic name for the compound represented below?
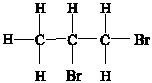
Provide complete and step by step solution for the question and show calculations and use formulas.