Assignment:
Q1. Name each of the following compounds.
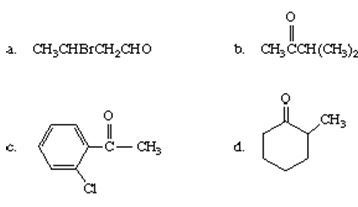
Q2. Write a structural formula for each of the following compounds.
a. benzyl methyl ketone
b. 2-pentenal
c. 2-chloro-3-hexanone
d. 2,3-dimethylpentanal
Q3. Write an equation for the synthesis of 2-hexanone by
a. oxidation of an alcohol
b. hydration of an alkyne
Q4. Write an equation for the reaction of propanal with each of the following reagents, and name the organic product.
a. cyanide ion
b. sodium borohydride
c. phenylmagnesium bromide, then H3O+
Q5. Using a Grignard reagent and the appropriate aldehyde or ketone, show how each of the following can be prepared.
a. 2-phenyl-2-butanol
b. 2-hexanol
c. 1-pentene-3-ol
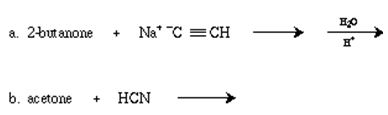
Q6. Complete the equation for the reaction of
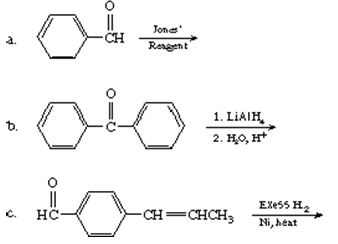
Q7. Give the structure of each product.
Q8. Write out the steps for the aldol condensation reaction between the enolate anion of propanal with pentanal.
Q9. Draw the Fisher projection formulas for the following:
a. L-ribose
b. L-arabinose
c. L-glucose
d. L-talose
Q10. Draw the Fisher projection formulas for the following and then convert them to three-dimensional representations.
a. L-threose
b. L-erythrose
Provide complete and step by step solution for the question and show calculations and use formulas.