1. (i) Draw Newman projections for the 6 rotational isomers of 3-methylpentane visualizing down the C2-C3 bond. (i) Draw the energy diagram for a 360 deg rotation about this bond, indicating the relative energies of the 6 species.
2. (i) Draw the two chair conformations for trans-2,3-dimethylcyclohexane. (ii) Draw the two chair conformations for cis-2,3-dimethylcyclohexane. (iii) Which isomer is more stable and why?
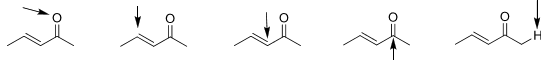
3. For the following, determine if the atoms (indicated by the arrow) are electrophilic, nucleophilic, or neither.
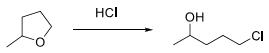
4. Propose reasonable mechanism for the following transformation using curved arrow notation.
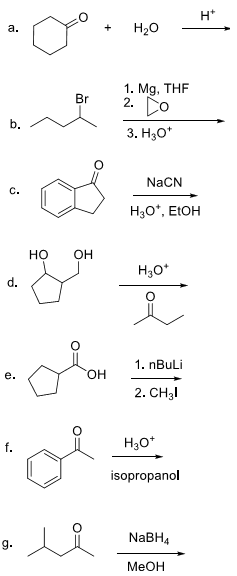
5. Provide the major product for the following reactions. Use curved arrow notation to indicate the flow of electrons.
6. (i) Draw a mechanism using curved arrow notation for the acid-catalyzed cleavage (hydrolysis) of the following molecule.
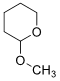
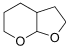
(ii) Synthesize the following molecule using the appropriate carbonyl compound.
7. The beta-blocker atenolol is commonly prescribed for high blood pressure and is used to slow the heart rate of patients at risk for "a-fib". The molecular structure is shown below.
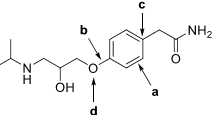
a. What is the molecular formula?
b. Circle and name all functional groups present.
c. Provide bond descriptions for bonds a and b.
d. Give the hybridization of carbon c and oxygen d.
e. Explain how the hybridization of carbon c occurs.
f. Draw the VB representation (orbital overlap) of the bonds shown below.
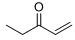
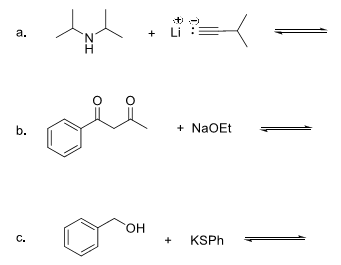
8. For the following acid base reactions, (a) write the expected products, (b) label all acids and bases and their conjugates, (c) estimate the pKa values, and (c) predict the direction of the equilibrium.
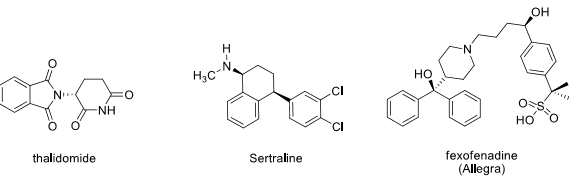
9. In the following chiral pharmaceuticals, indicate ALL stereocenters with an asterisk (*) and assign the absolute configuration (R or S).
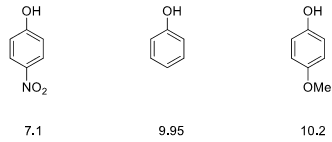
10. Explain the following observation. Be sure to include resonance structures in your discussion and use structures where appropriate.