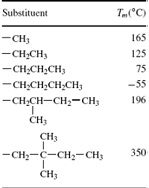
Response to the following problem:
The melting points of a series of poly(α-olefin) crystals were studied. All of the polymers were isotactic and had chains substituents of different bulkiness. The results are listed below. Derive a relationship between the melting point, Tm, and the enthalpy and entropy of fusion, _Hf and _Sf , respectively.
Use this relationship, plus what you know about polymer crystallinity and structure, to rationalize the trend in melting point.