Assignment:
Nicotine in a water solution containing 2% nicotine is to be extracted with kerosene at 293 K. Water and kerosene are essentially insoluble. Determine the percentage extraction of nicotine if 100 kg of the feed solution is extracted in a sequence of four batch ideal extractions using 49.0 kg of fresh, pure kerosene each. The equilibrium data are as follows (Claffey et al., 1950)
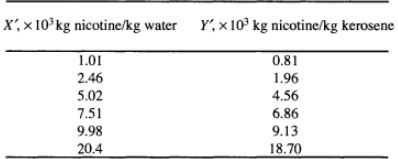
Provide complete and step by step solution for the question and show calculations and use formulas.