The potential energy between two atoms can be represented as follows:
V = - A/r + B/r10
Where A and B are constants and r the interatomic separation distance. Plot the potential energy verses distance relationships for these atoms when A= 5 x 10-30 J.m and B= 8 x 10-121 J.m10. Determine the coefficient of thermal expansion (in m/m.K) for the material over the following temperature ranges:
a) 0oK to 0oC,
b) 0oC to 820oC.
assume that thermal energy of the system is 3kT/2 where k is Boltzman's constant k = 1.381 x10-23 J/K.
2. Calculate the density of BaTiO3. It has the perovskite structure. In the perovskite structure, the Ba2+ and O2-combine to form a cubic close packed structure with the Ba2+ on the cube corners and the O2- in the face centre positions (because the Ba2+ and O2- have nearly the same radius). The small Ti4+ fits nicely in an octahedral site. Because of stoichometry and charge neutrality, only ¼ of the octahedral sites (those in the body centre position) are filled with Ti4+. See figure below.
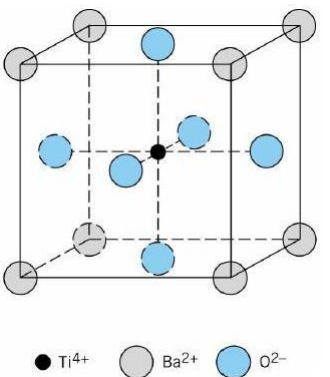
Ionic Radius Atomic Mass
Ba2+ 1.36 Ångstroms 137.3 g/mole
Ti 4+ 0.61 Ångstroms 47.9 g/mole
O2- 1.40 Ångstroms 16.0 g/mole
Avogadro's number (NAv) is 6.02 x 1023 atoms per mole.
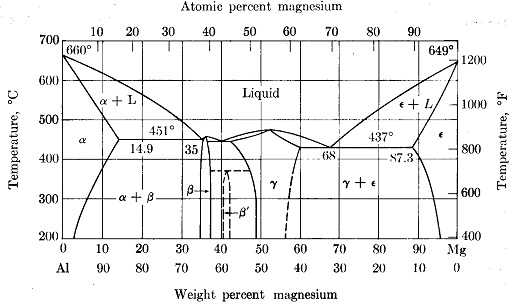
3. Using the phase diagram below for the aluminium-magnesium system, determine
i. the phases present and
ii. the composition (wt% Mg) of each phase and
iii. the amount of each phase for:
a) an aluminium-magnesium (5 wt % Mg) alloy at 500 oC.
b) an aluminium-magnesium (20 wt % Mg) alloy at 550 oC.
c) an aluminium-magnesium (80 wt % Mg) alloy at 300 oC.