Assignment:
(1) Solution A and B are separated by a membrane that is permeable to Mg2+ and impermeable to C1-. Solution A contains 0.1 mM MgC12. solution B contains 100 mM MgC12.
Mgt+ will be at electrochemical equilibrium when solution A is around_____ mV.
A. + 180 mV
B. - 180 mV
C. + 90 mV
D. - 90 mV
E. + 60 mV
F. - 60 mV
(2) Refer to the figure showing changes in membrane potential graphed during an action potential production. For step 3 in the figure,
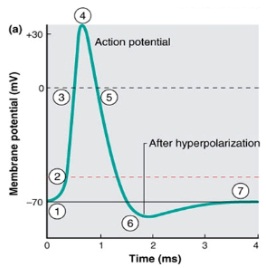
A. Voltage-gated K+ channels are open.
B. Voltage-gated Na+ channels are open.
C. Voltage-gated Ca2+ channels are open.
D. Voltage-gated Mg2+ channels are open.
E. Voltage-gated C1-hannels are open.