Assignment:
The activated carbon leaving the adsorber of Problem 3.16 is regenerated by countercurrent contact with steam at 380 K and 1 atm. The regenerated carbon is returned to the adsorber, while the mixture of steam and desorbed benzene vapors is condensed. The condensate separates into an organic and an aqueous phase and the two phases are separated by decantation. Due to the low solubility of benzene in water, most of the benzene will be concentrated in the organic phase, while the aqueous phase will contain only traces of benzene
(a) Calculate the minimum steam flow rate required.
(b) For a steam flow rate 50% above the minimum, calculate the benzene concentration in the gas mixture leaving the desorber, and the number of ideal stages required.
The equilibrium adsorption data at 380 K are as follows:
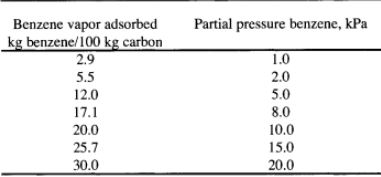
Provide complete and step by step solution for the question and show calculations and use formulas.