Assignment:
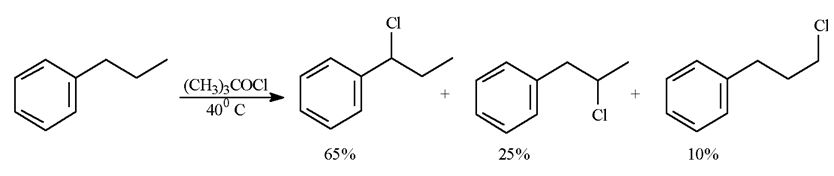
Q1. Refer to above reaction. When propylbenzene reacts with tert-butylhypochlorite three monochlorinated products are formed in the ratios indicated. Calculate a reactivity order for each type of hydrogen atom in propylbenzene.
Q2. Explain why (1-chloropropyl) benzene is the major product of this reaction
Q3. Will the product mixture of this reaction display optical activity?
Provide complete and step by step solution for the question and show calculations and use formulas.