Solve the following problem:
Take an ideal monatomic gas γ = 5/2
Around the Carnot cycle, where point 1 at the beginning of the adiabatic compression has pressure P1 = Po (atmospheric pressure), volume V1 = 13 liters, and temperature T1 = 300 K. Point 3 has pressure P3 = 2Po and volume V3 = 26 liters.
The resulting Carnot cycle is shown in Fig. (A). Calculate the values of volume and pressure at all four points, which have the same meaning as those in Fig. (B).
Fig.(A):
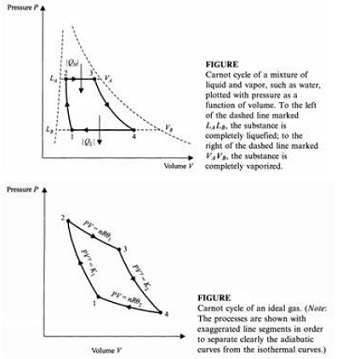 ←Fig.(B)
←Fig.(B)