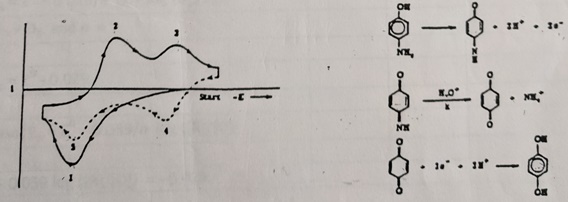
Problem 1: The cyclic voltammogram and reaction scheme for p-aminophenol is given in Figure 1. Identify each peak (labeled 1-5) in the cyclic voltammogram with the reduction or oxidation of species.
Problem 2: Chronoamperometry is frequently used to measure diffusion coefficients of electroactive species and surface areas of working electrodes. The following chronoamperometric data were obtained during the oxidation of 0.2 mM ferrocyanide solution at a glassy carbon electrode. The diffusion coefficient for ferrocyanide is 6.3 x 10-6 cm2/s.
a. Calculate the area of the glassy carbon electrode
b. Do the data indicate planar diffusion?
|
Time, s
|
i, µA
|
|
5
|
7.18
|
|
8
|
5.55
|
|
11
|
4.76
|
|
15
|
4.53
|
|
18
|
3.78
|
|
21
|
3.5
|
Problem 3: The E0' and n of a metal complex can be determined by the optically transparent thin-layer electrode (OTTLE) technique. The spectra in figure 3 were obtained at potentials indicated on the figure. Draw the appropriate Nenstian plot and calculate E0' and n for the complex.
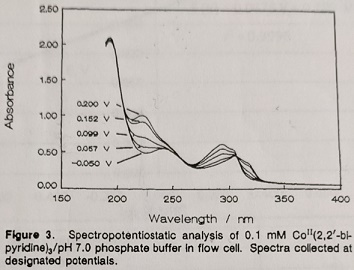
[DeAngelis, T.P. Heineman, W.R. J. Chem. Educ. 1976, 53, 594.]
Take a break from your regular hectic-schedule and approach our Chronoamperometry Assignment Help service for getting the most un-matched online assistance!
Tags: Chronoamperometry Assignment Help, Chronoamperometry Homework Help, Chronoamperometry Coursework, Chronoamperometry Solved Assignments, Cyclic Voltammogram Assignment Help, Cyclic Voltammogram Homework Help