Assignment:
Consider the bromination of the compound 2-methyl 1-pentene
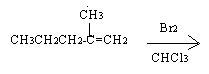
Q1. Write the basic structure of the product of the bromination of 2-methyl 1-pentene, without regard to stereochemistry.
Q2. Does the product have any chiral carbons? If so, identify them with an circle.
Q3. How many stereoisomers are formed from the bromination of 2-methyl 1-pentene?
Q4. Draw Fischer projections of the stereoisomers formed in the bromination of 2-methyl 1-pentene and name each, including an "R" or "S" designation for the configuration of each stereoisomer.
Provide complete and step by step solution for the question and show calculations and use formulas.