Assignment:
Q1. For the reactions:
Cl + H-CH3 --> Cl-CH3 + H
Cl + H-CH3 --> CH3 + H-Cl
a) Calculate the change in H for each reaction using bonds dissociation enthalpies.
b) One of these reactions is the rate-determining step in the correct mechanism for the chlorination of methane. What is it? Explain why, based on the change in H values you calculated.
Q2. Consider the reaction scheme.
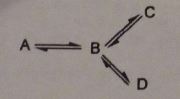
The conversion of A to B is endothermic by 30 kcal/mol (125.5 kJ/mol) and the conversion of B to C and B to D are exothermic by 35 and 40 kcal/mol (146.4 and 167.4 kJ/mol), respectively. The energies of activation are as follows: B to A, 6 kcal/mol (25 kj/mol); B to C, 4 kcal/mol (16,8 kJ/mol); and B to D, 2 kcal/mol (8.4 kJ/mol).
a) Draw a complete labeled energy-profile diagram for this reaction scheme. Indicate the location of the transition states and calculate the heights of all the energy barriers that separate A to D.
b) Indicate the rate determining step(s) in the scheme.
Provide complete and step by step solution for the question and show calculations and use formulas.