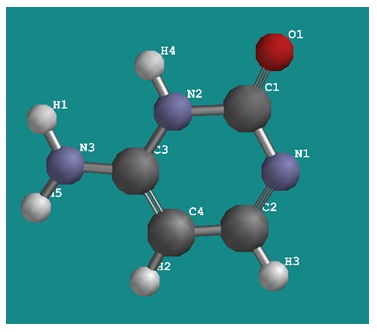
Cytosine is one of the four bases which are used in the genetic code. It has the molecular formula C4H5N3O. The molecular structure of cytosine computed using Spartan with a 3-21G* basis set is displayed below.
Question 1: Draw an electron-dot (valence bond) structure of cytosine. Display all the lone pairs. Using your electron-dot structure, predict the structure and hybridization of the heavy atoms. Use your structure to describe why cytosine is categorized as a base.
Question 2: Calculations and experiments like X-ray crystallography show that cytosine possesses a plane of symmetry. That is, all the atoms lie in the same plane.
a) What does the molecular planarity imply regarding the hybridization of the heavy atoms?
b) Where are the lone pairs on the N and O atoms, in the plane of the molecule or perpendicular to the plane?
c) Is the hybridization adequate to describe global planarity? Describe.
Question 3: Draw a resonance structure for cytosine. Which of the two resonance structures that you have drawn makes the bigger contribution to the wave function? Describe why.
Question 4: The given bond lengths in Å were measured in compounds in which there is only one electron-dot structure, that is, no resonance: C-C, 1.54; C=C, 1.32; CΞC, 1.20; C-N, 1.47; C=N, 1.26; CΞN, 1.16; N-N, 1.47; N=N, 1.25; NΞN, 1.10; C-O, 1.43; C=O, 1.20.
The given bond lengths in Ångstrom were determined from the Spartan structure of cytosine: C1-N1, 1.37; N1-C2, 1.30; C2-C4, 1.41; C4-C3, 1.37; C3-N2, 1.35; N2-C1, 1.42; C3-N3, 1.35; C1-O1, 1.21. Interpret such cytosine data by using your resonance structures.
Question 5: Cytosine has some isomers. Draw the structures of those isomers with similar ring structure, that is, the carbon atoms and two of the nitrogen atoms lie in a 6-membered ring. Consideration of such isomers was significant in the elucidation of the structure of DNA by Watson and Crick.