Assignment:
Photochromisim
Question 1: In the reaction of
4-Nitrobenzaldehyde + Acetophenone → trans-4-Nitrochalcone
a. The yield of the reaction to form your chalcone is not quantitative. What side products might be formed from your aldol condensation?
b. If one uses benzaldehyde rather than p-nitrobenzaldehyde in the aldol reaction the yield is extremely low, if it works at all. Offer an explanation for this result.
Question 2:
In the bromination of
trans-4-Nitrochalcone → erythro 2,3 dibromo 3-(4-nitrophenyl) propiophenone
a racemic mixture forms
a. Draw the structure of the enantiomers you form and assign R, and S to each of the stereocenters.
b. Will you see 2 spots on a TLC of your reaction products? Why or why not?
c. Is your bromination reaction stereoselective? Why or why not?
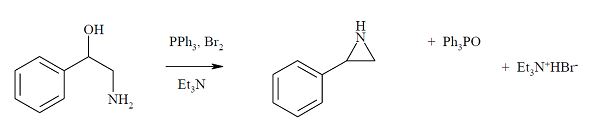
Question 3: Another route to the effective synthesis of aziridines is through the ring closure of a β-amino alcohol in the presence of triphenylphosphine and bromine. Prove an arrow-pushing mechanism for this reaction.
Question 4: When you had to form a photochromic imine you had to use ammonium bromide instead of HBr. Give 2 reasons why you cannot use concentrated HBr.
Provide complete and step by step solution for the question and show calculations and use formulas.