1. The potential energy between two atoms can be represented as follows:
V = -A/r + B/r10
Where A and B are constants and r the interatomic separation distance. Plot the potential energy verses distance relationships for these atoms when A= 8 x 10-30 J.m and B= 5 x 10-119 J.m10. Determine the coefficient of thermal expansion (in m/m.K) for the material over the following temperature ranges:
a) 0oK to 0oC,
b) 0oC to 820oC.
assume that thermal energy of the system is 3kT/2 where k is Boltzman's constant k = 1.381 x10-23 J/K.
2. Gallium arsenide (GaAs) has the zinc blende structure. The gallium atoms are in FCC packing and the arsenic atoms occupy half the tetrahedral sites. Calculate the density of Gallium arsenide (GaAs) using the following data.
Covalent Radius Atomic Mass
Ga 1.26 Ångstroms 69.7 g/mole
As 1.19 Ångstroms 74.9 g/mole
Avogadro's number (NAv) is 6.02 x 1023 atoms per mole.
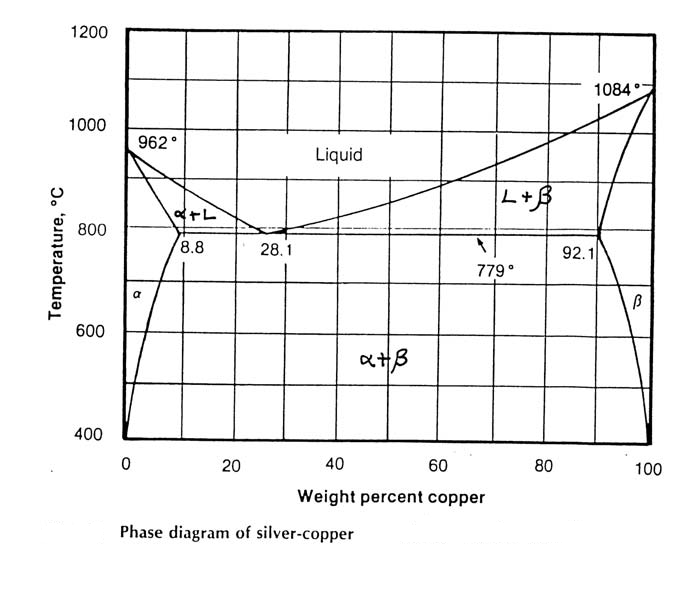
3. Using the phase diagram below for the copper-silver system, determine:
i) the phases present
ii) the composition (wt % Cu) of those phases and
iii) the amount (wt %) of each phase for
a) a copper-silver alloy (70 wt % copper) at 900oC
b) a copper-silver alloy (70 wt % copper) at 600oC
c) a copper-silver alloy (5 wt % copper) at 800oC