These types of polymers are formed as a result of condensation reaction between monomer units. Some common examples are being discussed here:
1. Polyesters
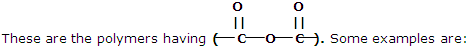
(i) Terelene: It is a polymer obtained by the condensation reaction between ethylene glycol and terephthalic acid
Characteristics and uses: Terelene is resistant to the action of chemical and biological substances and also to abrasion. It has a less moisture absorbing capacity. As such it is widely used in making wash and wear fabrics. The polyster textile fabrics made from the polymer are marketed under the trade name Terelene or Dacron.
It is used as a blend with cotton and wool in clothing. It is also utilized in seat belts and sails. The polymer is also used as mylar in the preparation of films, magnetic recording tapes and for packing frazen food. Dacron (and Teflon) tubes are god substitutes for human blood vessels in heart by-pass operations.
(ii) Glyptic or Alkyd Resin: Glyptal is a common name of all polymers prepared by condensation of di-basic acids, and polyhydric alcohols. The simplest glyptal is poly (ethylene glycol phthalate) which is obtained by the condensation of ethylene glycol and pthlatic acid.
Characteristics and uses: these are three dimensional cross-linked polymers. Poly (ethylene glycol phthalate) dissolves in suitable solvents and the solution on evaporation leaves a tough and non-flexible film. Therefore, it is utilized in:
2. Polyamides

1. Nylon-6, 6: It is a polymer of adipic acid (1, 6-hexaneioic acid) and hexamethylene diamine (1, 6-diaminohexane)

Characteristics and uses: Nylon-66 (read as nylon-six-six) can be cast into a sheet or fibres by spinning devices. Nylon fibres generally have high tensile strength. They are hard and resistant to abrasion. They are also somewhat elastic in nature. Nylon finds uses in: producing bristles and brushes, carpets and fabrics in textile industry, elastic hosiery in the shape of crinkled nylon.
2. Nylon 6, 10: It is a polymer of hexamethylene diamine (six carbon atoms) and sebacoyl chloride (ten carbon atoms)
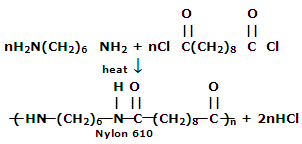
3. Nylon-6 (or Perlon): It is prepared from the monomer caprolactum. Caprolactum is obtained from cyclohexane according to the reaction sequence given below:
Caprolactum on heating with traces of water hydrolyses to 6-amino caproic acid which on continued heating undergoes self-condensation and polymerises to give nylon-6.
Nylon-6 finds uses in the manufacture of tyre cords, fabrics and ropes.