Two tanks which contain water are connected to each other through a valve. The initial conditions are as shown (at equilibrium):
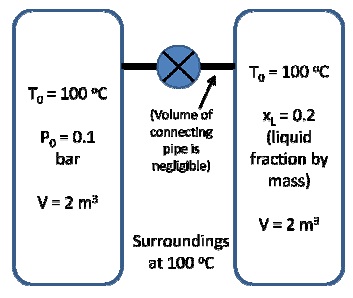
Note that in the tank on the right we have vapor-liquid equilibrium. The valve is opened, and the two tanks equilibrate. However, the tanks are not well insulated and so the tanks achieve However, the tanks are not well insulated and so the tanks achieve However, the tanks are not well insulated and so the tanks achieve thermal equilibrium with the surroundings as well.
a) What is the final pressure of the tanks?
b) How much heat is transferred to or from the surroundings by allowing the tanks to mix? Full credit will require the correct sign (+/-).