The following mixture of hydrocarbons is obtained as one stream in a petroleum refinery.
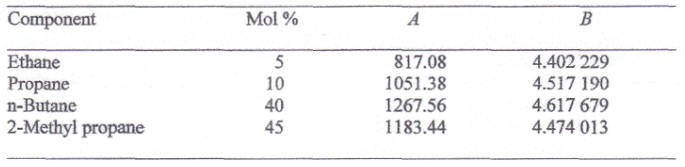
The parameters A and B in this table are the constants in the equation
log10 Pvap = (-A/T) + B
Where Pvap is the vapor pressure in bar and T is the temperature in K. These paraffinic hydrocarbons form an ideal mixture.
a. Compute the bubble point of me mixture at 5 bar.
b. Compute the dew point of the mixture at 5 bar.
c. Find the amounts and compositions of the vapor and liquid phases that would result if this mixture were isothermally flash-vaporized at 30°C from a high pressure to 5 bar.