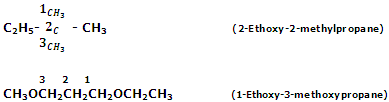Common system
According to this system, the individual members are named according to alkyl groups attached to the oxygen atom. The two alkyl or aryl groups linked oxygen atom are named in the alphabetic order followed by the word ether. In case of simple ethers, the prefix is attached before the name of the alkyl group. Some instances are as listed below:
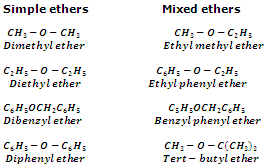
IUPAC system
According to IUPAC system, ethers are named as alkoxyalkanes. The larger alkyl group forms the part of parent chain while lower alkyl group constitutes alkaxy radical. Various examples are given below:
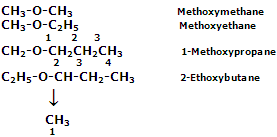
The IUPAC names of some more ethers are being given as follows: